50090-2399 : Ethambutol Hydrochloride 400 mg Oral Tablet, Film Coated
| NDC: | 50090-2399 |
| Labeler: | A-s Medication Solutions |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Ethambutol Hydrochloride Ethambutol Hydrochloride |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA016320 |
| Rev. Date: |
Appearance:
| Markings: | E7 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 13 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
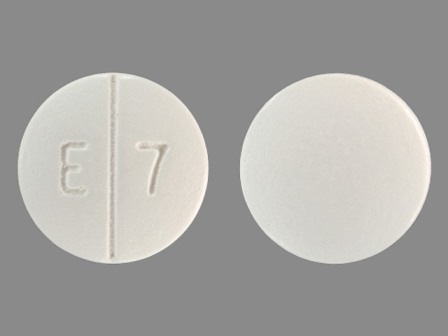
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 50090-2399-0: 100 TABLET, FILM COATED IN 1 BOTTLE (50090‑2399‑0)
Active Ingredients:
- Ethambutol Hydrochloride
Dosage Strength:
- 400 mg
Inactive Ingredients:
- Gelatin
- Hydroxypropyl Cellulose (Type H)
- Magnesium Stearate
- Sodium Lauryl Sulfate
- Sorbitol
- Stearic Acid
- Sucrose
- Titanium Dioxide
Pharmaceutical Classes:
- Antimycobacterial [EPC]
Related Products:
Based on records with the same trade name.- 50090-2337 Ethambutol Hydrochloride 100 mg Oral Tablet, Film Coated by A-s Medication Solutions
- 50090-3948 Ethambutol Hydrochloride 400 mg Oral Tablet, Film Coated by A-s Medication Solutions
- 50090-5140 Ethambutol Hydrochloride 100 mg Oral Tablet by A-s Medication Solutions
- 50090-5142 Ethambutol Hydrochloride 400 mg Oral Tablet by A-s Medication Solutions
- 0555-0923 Ethambutol Hydrochloride 400 mg Oral Tablet by Barr Laboratories Inc.
- 42806-101 Ethambutol Hydrochloride 100 mg Oral Tablet, Film Coated by Epic Pharma, LLC
- 42806-102 Ethambutol Hydrochloride 400 mg Oral Tablet, Film Coated by Epic Pharma, LLC
- 49349-065 Ethambutol Hydrochloride 100 mg Oral Tablet by Remedyrepack Inc.
- 49349-446 Ethambutol Hydrochloride 400 mg Oral Tablet by Remedyrepack Inc.
- 52125-012 Ethambutol Hydrochloride 100 mg Oral Tablet by Remedyrepack Inc.
- 53002-6580 Ethambutol Hydrochloride 400 mg Oral Tablet by Rpk Pharmaceuticals, Inc.
- 53808-0977 Ethambutol Hydrochloride 400 mg Oral Tablet by State of Florida Doh Central Pharmacy
- 54879-001 Ethambutol Hydrochloride 100 mg Oral Tablet by Sti Pharma LLC
- 54879-002 Ethambutol Hydrochloride 400 mg Oral Tablet by Sti Pharma LLC
- 55695-023 Ethambutol Hydrochloride 100 mg Oral Tablet, Film Coated by Department of State Health Services, Pharmacy Branch
- 55695-035 Ethambutol Hydrochloride 400 mg Oral Tablet, Film Coated by Department of State Health Services, Pharmacy Branch
- 58118-0003 Ethambutol Hydrochloride 400 mg Oral Tablet by Clinical Solutions Wholesale, LLC
- 61786-359 Ethambutol Hydrochloride 400 mg Oral Tablet, Film Coated by Remedyrepack Inc.
- 68084-280 Ethambutol Hydrochloride 400 mg Oral Tablet by American Health Packaging
- 68151-0149 Ethambutol Hydrochloride 100 mg Oral Tablet by Carilion Materials Management
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 50090-2398Next: 50090-2401 >
Related Discussions:
myrin p ethambutol hci rifampicin isoniazid pyramids
Hi Myrin P Ethambutol Hci Rifampicin Isoniazid Pyramids My misses got this medicines please can you explain to me what i... 2 replies
Hi Myrin P Ethambutol Hci Rifampicin Isoniazid Pyramids My misses got this medicines please can you explain to me what i... 2 replies
Pimples, are these side effects of ethambutol, isoniazid abd rifampicin?
I'm on my 5th month of taking those medicines. I dont actually had any pimple problem before especially on my face. ... 1 reply
I'm on my 5th month of taking those medicines. I dont actually had any pimple problem before especially on my face. ... 1 reply
fixcom 4 Film coated tablet Antituberculosis each tablet contains Rifampicin 150 mg Isoniazid 75mg Pyrazinamide 400mg Ethambutol 275mg
fixcom 4 Film coated tablet Antituberculosis each tablet contains Rifampicin 150 mg Isoniazid 75mg Pyrazinamide 400mg Et... 4 replies
fixcom 4 Film coated tablet Antituberculosis each tablet contains Rifampicin 150 mg Isoniazid 75mg Pyrazinamide 400mg Et... 4 replies
ethambutolpyrazinamrifampin is given for what
i was basically trying to see what this is prescribed for what illness...
i was basically trying to see what this is prescribed for what illness...
Ethambutol 400mg
colon cancer...
colon cancer...
ethambutol
...
...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.